mg+ electron configuration|mg electron configuration long form : Cebu Electron configuration of magnesium. In the periodic table, this element belongs to group IIA. It is very light and silvery-white in color and is considered the lightest structural metal . Aviator and Casino Games also on offer; Play on mobile or on the Easybet App; R50 Free Bet, 25 Free Spins & 150% deposit match up to R1000. Claim Offer Review . Aviator becomes a really fun game. .
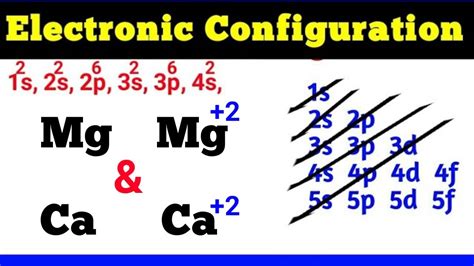
mg+ electron configuration,March 23, 2023. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.mg+ electron configurationClick on above elements (in Periodic table) to see their information or Visit .In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in . The arrangement of electrons in magnesium in specific rules in different orbits and orbitals is called the electron configuration of magnesium. The electron .
Electron configuration of magnesium. In the periodic table, this element belongs to group IIA. It is very light and silvery-white in color and is considered the lightest structural metal .
What is the Electron Configuration of Magnesium. Ne 3s2 is the electron configuration for Mg. Many other electrons configurations of other elements have .
Magnesium Electron Configuration. Wayne Breslyn. 753K subscribers. Join. Subscribed. 634. 143K views 10 years ago. A step-by-step description of how to .
FAQ. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change .The electron configuration of Magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of Magnesium is .
Get the facts about element Magnesium (Mg) [12] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data . Magnesium electron configuration notation [Ne]3s 2 is the electronic configuration notation of Mg, where it has 12 electrons out of which 10 electrons can be represented for Neon and remaining two electrons in 3s. Magnesium unabbreviated electron configuration. 1s 2 2s 2 2p 6 3s 2 is an unabbreviated electronic . The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron . Electron Shell Configuration: [Ne]3s 2; Characteristics. . Mg 25 and Mg 26 are used to study the absorption and metabolism of magnesium in the human body. They are also used to study heart disease. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. These . Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.The chemical symbol for Magnesium is Mg. Electron Configuration and Oxidation States of Magnesium. Electron configuration of Magnesium is [Ne] 3s2. Possible oxidation states are +2. Electron .2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to ..For example, the last electron added in the electron configuration of magnesium is the second 3s-electron. Mg + would have a configuration of 1s 2 2s 2 2p 6 3s 1. For Mg 2+, the electron configuration would be 1s 2 2s 2 2p 6. There is an exception to this process for transition metals (members of the d-block) and the lanthanoids and actinoids .The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3s orbital, giving a 1s 2 2s 2 2p 6 3s 1 configuration.

Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .mg electron configuration long form The electron configuration of Magnesium in terms of the shell or orbit is [2, 8, 2]. The ground-state electron configuration of the Magnesium (Mg) atom is 1s 2 2s 2 2p 6 3s 2. And for the excited state, it is 1s 2 2s 2 2p 6 3s 1 3p 1. The shorthand electron configuration for Magnesium is [Ne] 3s 2. The electron configuration for the .
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( .
So if you're thinking about the subshell, the s subshell could fit two electrons, the p subshell can fit six electrons, the d subshell can fit 10 electrons, and the f subshell can fit 14 .Write the electron configuration for a magnesium atom. Write the electron configuration for the element magnesium. A. 1s^2 2s^2 2p^8 B. 1s^2 2s^2 2p^6 3s^2 C. 1s^2 2s^10 D. 1s^2 2s^2 2p^6 3s^2 3p^2 E. 1s^2 2s^2. 2p^6 3s^1; Write the ground state electron configurations of the following transition metal ions. a. Sc3+ b. Cr3+ c. Cu+ d.Au+Its electron configuration is. He: 1s2 He: 1 s 2. The three electrons for Li are arranged in the 1s subshell (two electrons) and the 2s subshell (one electron). The electron configuration of Li is. Li: 1s22s1 Li: 1 s 2 2 s 1. Be has four electrons, two in the 1s subshell and two in the 2s subshell.
The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .

Electrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Magnesium is 12. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative .mg+ electron configuration mg electron configuration long formElectrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Magnesium is 12. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative . To write the orbital diagram for the Magnesium atom (Mg) first we need to write the electron configuration for just Mg. To do that we need to find the number.
mg+ electron configuration|mg electron configuration long form
PH0 · valence electrons chart
PH1 · mg electron configuration shorthand
PH2 · mg electron configuration long form
PH3 · how to write electron configuration
PH4 · electron configuration worksheet
PH5 · electron configuration of all elements
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa